RIVANNA Launches Accuro Second Generation With BoneEnhance® Multi-Frequency Image Reconstruction And SpineNav3D™ AI-Based Spine Recognition
CHARLOTTESVILLE, VA., March 23, 2021 — RIVANNA®, developer of world-first, imaging-based medical solutions, announces the market introduction of Accuro Second Generation, combining innovative thoracic spine functionality with its groundbreaking introductory system. The new Accuro spinal navigation system features BoneEnhance® Multi-Frequency Image Reconstruction, which enhances bone-to-tissue contrast for improved landmark and needle visualization, and SpineNav3D™ AI-Based Spine Recognition, which automates spinal landmark identification and depth measurements during spinal needle guidance procedures.
 The novel imaging algorithms are part of the company’s growing patent portfolio covering its core technologies. BoneEnhance Multi-Frequency Image Reconstruction improves visualization of lumbar and thoracic bony anatomy and compensates for ultrasound’s inherent distortion with a 5- to 10-fold increase in bone-to-tissue contrast. Contributing to image quality is patented bone-specific ultrasound-probe technology, limiting the bone artifacts that detract from image contrast and clarity in conventional systems. SpineNav3D AI-Based Spine Recognition facilitates image interpretation of individual 2D lumbar and thoracic spine scans by automating spinal bone landmark detection and depth measurements and providing a real-time assessment of the scan plane orientation in 3D.
The novel imaging algorithms are part of the company’s growing patent portfolio covering its core technologies. BoneEnhance Multi-Frequency Image Reconstruction improves visualization of lumbar and thoracic bony anatomy and compensates for ultrasound’s inherent distortion with a 5- to 10-fold increase in bone-to-tissue contrast. Contributing to image quality is patented bone-specific ultrasound-probe technology, limiting the bone artifacts that detract from image contrast and clarity in conventional systems. SpineNav3D AI-Based Spine Recognition facilitates image interpretation of individual 2D lumbar and thoracic spine scans by automating spinal bone landmark detection and depth measurements and providing a real-time assessment of the scan plane orientation in 3D.
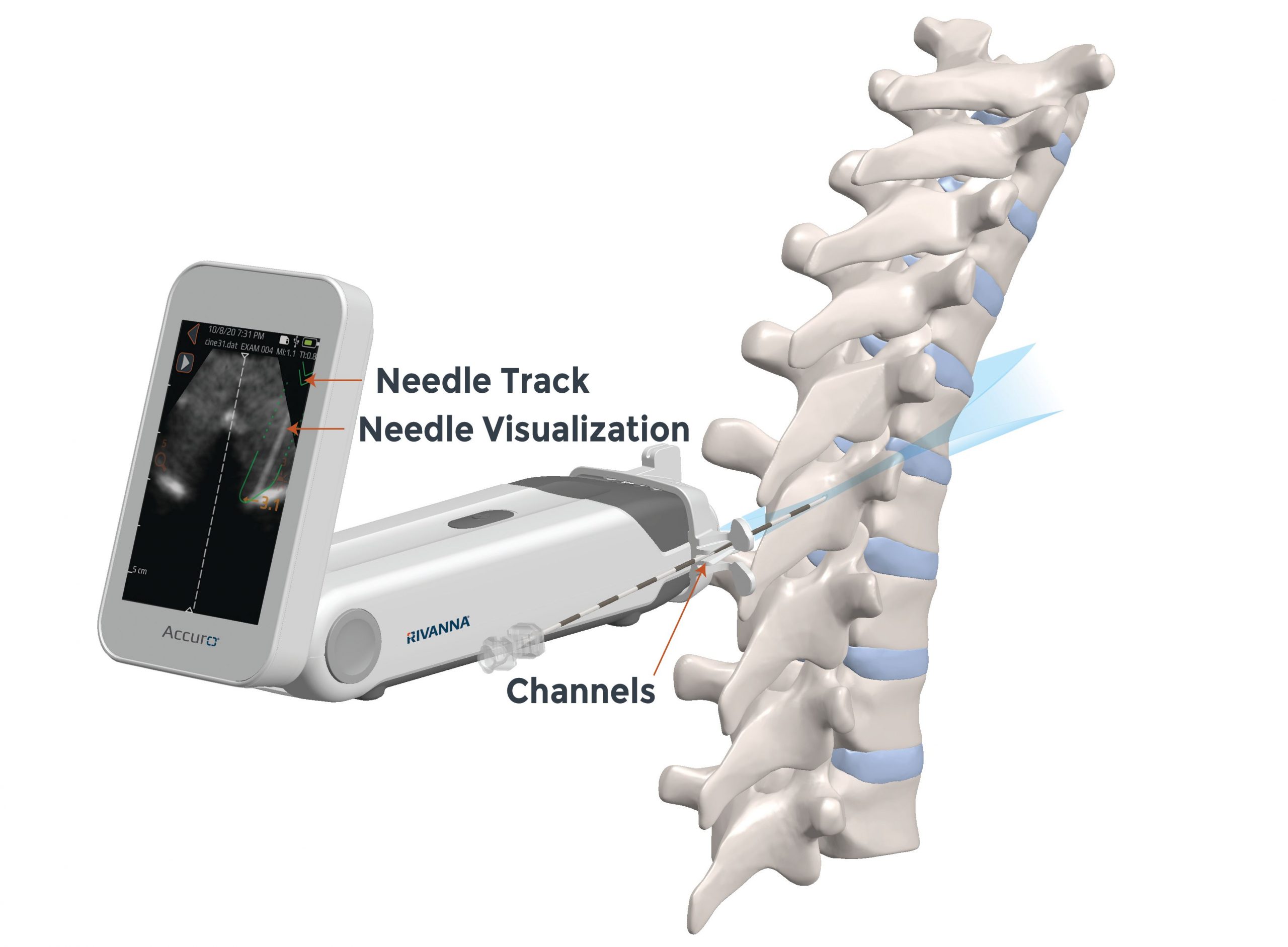
Accuro’s new Thoracic presetting utilizes SpineNav3D AI-based pattern recognition with customized anatomical indicators that display the appropriate needle-track trajectory for thoracic paramedian epidural procedures. Real-time needle-track and interlaminar depth indicators overlay the BoneEnhance image once spinal landmark anatomy is detected at a location enabling paramedian needle access to the interlaminar space. Needle enhancement allows real-time visualization of the Tuohy progressing along the prescribed needle-track pathway, helping anesthesia providers avoid the involvement of vulnerable nearby anatomy.
Will Mauldin, PhD, co-founder, and CEO of RIVANNA, commented, “Accuro is filling a need in the healthcare delivery stream via an easy-to-use device that enables anesthesia providers with little or no ultrasound experience the ability to improve procedural efficacy and patient safety. Users can take advantage of precise image guidance rather than relying on traditional palpation to estimate the spinal midline and appropriate intervertebral space for needle placement. Currently, there are no modalities like Accuro for anesthesia providers, specifically designed to improve the efficiency of spinal needle guidance procedures.”
Development of Accuro’s new technologies for thoracic epidural placement and validation through clinical study was sponsored by the National Institute of General Medical Sciences of the National Institutes of Health; this included the review of more than 50,000 images collected at Memorial Sloan Kettering Hospital under principal investigator Amitabh Gulati, MD, which were rigorously tested, optimized and integrated into Accuro’s new Thoracic preset.

About RIVANNA
RIVANNA is a privately held designer, manufacturer, and distributor of world-first, imaging-based medical solutions based in Charlottesville, VA. RIVANNA operates an FDA-registered and ISO 13485:2016 certified manufacturing facility where it produces the Accuro product line and related medical equipment and components. Accuro is the world’s first spinal navigation device designed to improve the safety, speed and efficiency of spinal needle guidance procedures. RIVANNA’s revolutionary platforms feature BoneEnhance, Multi-Frequency Image Reconstruction, which optimizes ultrasound for the visualization of bony versus soft tissue anatomy, and SpineNav3D AI-Based Spine Recognition, which automates ultrasound image interpretation.