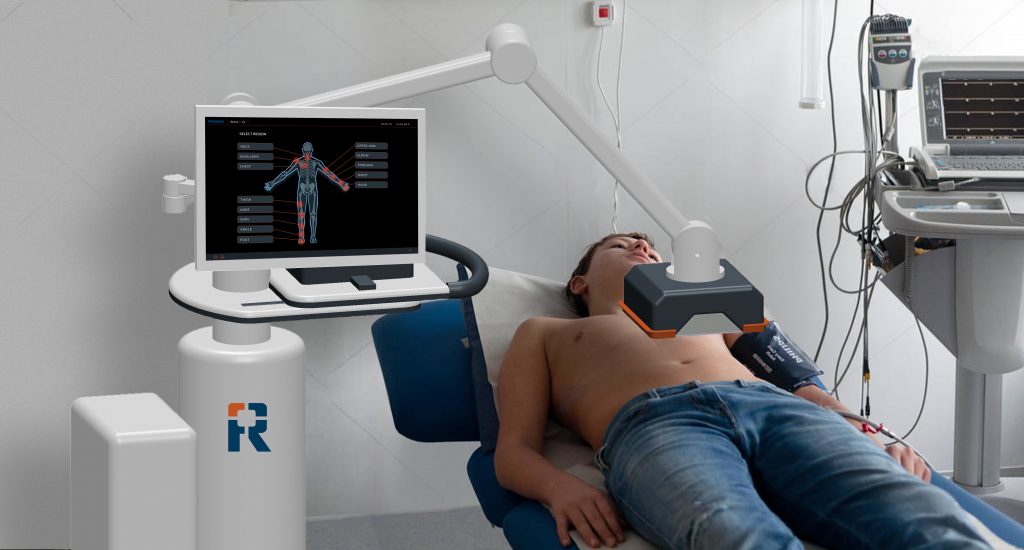
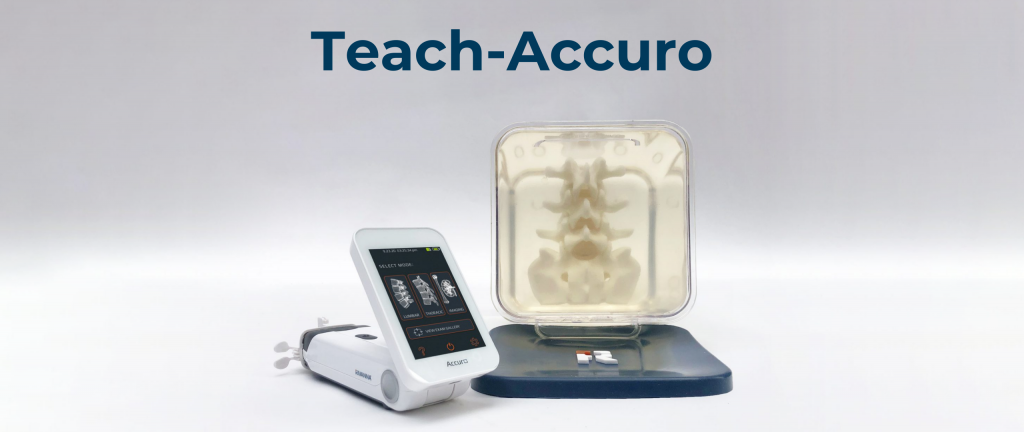
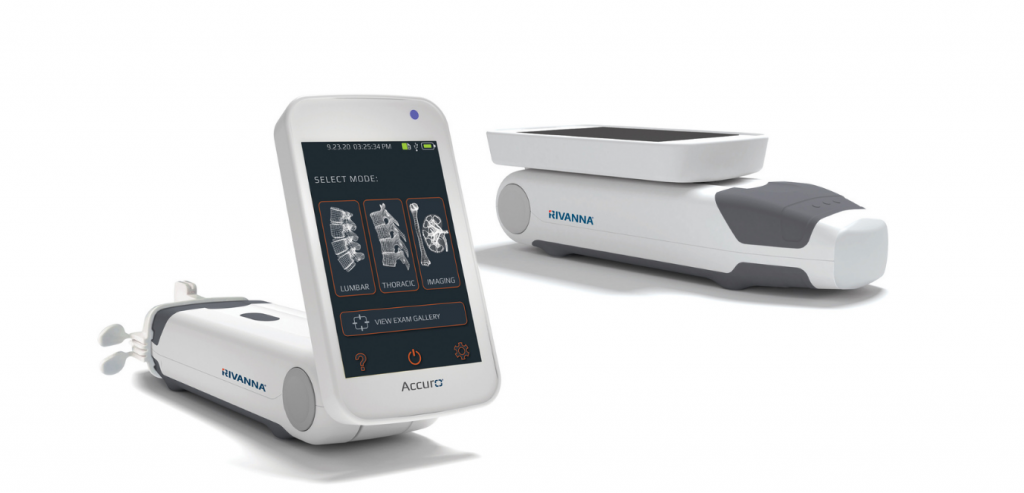
RIVANNA announces FDA clearance of Accuro 3S Needle Guide Kit
CHARLOTTESVILLE, VA, DECEMBER 16, 2025 — RIVANNA®, developer of world-first imaging-based medical technologies, today announced that the U.S. Food and Drug Administration (FDA) has granted 510(k) clearance for its Accuro® 3S Needle Guide Kit consumables. This...